|
|
Original Article
| ||||||
| A novel methodology for quantitative analysis of different phenotypes of locomotion behavior: C. elegans bioinformatics model | ||||||
| Keyur Vora1, Bill Walthall1, Gennady Cymbalyuk2 | ||||||
|
1Department of Biological Sciences, Georgia State University, Atlanta, GA, USA 2Department of Neurosciences, Georgia State University, Atlanta, GA, USA | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar articles in PubMed] [Similar articles in Google Scholar] |
| How to cite this article |
| Vora K, Walthall B, Cymbalyuk G. A novel methodology for quantitative analysis of different phenotypes of locomotion behavior: C. elegans bioinformatics model. Edorium J Cell Biol 2018;4:100008C06KV2018. |
|
ABSTRACT
|
|
Aims: Genetic makeup plays an important role in producing variety of anatomical structures and locomotion patterns accordingly. It is very challenging to define any genetic basis for the generation of a functional motor output during locomotion, formed by the interaction between neural networks. Hence, detecting locomotion pattern under different genetic environments is an important area of biological research. The main focus of this research is development of novel quantitative methods which are suitable for detecting locomotion behavior of wildtype and mutant forms of C. elegans. Methods: Wildtype and cnd-1 strains were studied using Q-imaging fluorescent microscope for worm localization, image filtering and video capturing data collection. All data were subjected to MATLAB image processing algorithm for quantitative and graphical analysis. Results: Wildtype animals were used as the standard for comparison as they show symmetric movements, with no favoring of either axis. In contrast cnd-1 shows more activity in markers near head and tail on dorsal side as well as central markers on ventral side. This neuromuscular coordination creates dorsal asymmetry in cnd-1 mutant forms. Conclusion: The algorithms and codes developed in this study provide a versatile tool to biologists interested in deciphering the fundamental molecular mechanisms of the locomotion behavior among diverse population of worms. The codes developed are likely to assist biologists efficiently in deriving useful quantitative information on the motility behavior of microorganisms. Keywords: C. elegans, MATLAB bioinformatics, Quantitative locomotion analysis |
|
INTRODUCTION
|
|
Locomotion confers a competitive advantage to living organisms by allowing them to navigate in the environment and reach the most favorable niches for growth and survival. Basically, the locomotion is defined as a natural event that involves a change in the position or location of the organism. In general, animals have a range of locomotion behavior from snakelike sinusoidal movement, rabbit like jumping, catlike walking or running to birdlike flying. This movement is the result of complex neuromuscular circuit activity that causes alternate contractions of muscles along the body walls [1]. Genetic makeup plays an important role in producing variety of anatomical structures and locomotion patterns accordingly. Also there are common principles lying behind different neural circuitry in different animals responsible for producing particular locomotion pattern. It is very challenging to define any genetic basis for the generation of a functional motor output during locomotion, formed by the interaction between neural networks, local feedback from sensory neurons about movements and forces generated in the locomotor organs and coordinating signals from neighboring segments or appendages [2]. Hence, detecting locomotion pattern under different genetic environments is an important area of biological research. According to biologists, an essential prerequisite for studying locomotion and neural circuit mechanism is the development of quantitative methods that enable the characterization of subtle and transient changes in motility behavior of mutant forms. Making kinematic movies for observation and analysis is an important approach to study different locomotion patterns. Image processing can be utilized successfully to aid researchers in this area to understand and uncover many hidden facts and properties. Researchers from Department of Biology in Georgia State University used a special tool called ‘QIMAGING Fluorescent Video Microscope’ to capture the worms’ locomotion in successive time frames and with different mutant forms. The target worms and mutants chosen for this research are Caenorhabditis elegans known as C. elegans, a nematode or roundworm, the first animal to have its genome sequenced and almost all the genes characterized [3]. With a mapped genome and the only mapped neural circuitry, this organism offers a first tangible opportunity to understand an entire living, behaving and learning system bottom-up and top-down. Also it is more amenable to different types of mutations that give us an opportunity to reproduce different mutant forms. Nematode sinusoidal movement has been used as a phenotype in many studies of C. elegans development, behavior and physiology. A thorough understanding of the ways in which genes control these aspects of biology depends, in part, on the accuracy of phenotypic analysis. While worms that move poorly are relatively easy to describe, description of hyperactive movement and locomotion modulation presents more of a challenge. An enhanced capability to analyze all the complexities of C. elegans locomotion will thus aid our understanding of how genes control behavior [4]. As such, it offers great promise to systems biologists, neuroscientists and computer scientists alike. Studies have been reported for automated tracking system of locomotor behavior using infrared micro beam scattering [5]. While the basic size and speed of C. elegans is readily observable, accurate quantitative measurements of these and other attributes of locomotion have only recently become possible. Hirose et al. (2003) have used computer-based image analysis to measure the C. elegans body automatically, and they reported an average length of 1.4 mm and a diameter of 0.08 mm in wild-type adults [6]. Feng et al. (2004) later developed a tracking and imaging system for automated quantitative analysis of videos of C. elegans locomotion. Using this system [7], Cronin et al. (2005) reported mean velocities of 0.2 mm/s and mean body wave amplitudes of 20% of the body length for wild-type adults [4]. The main focus of this research has been on developing methods which are suitable for detecting locomotion behavior of wildtype and mutant forms of C. elegans. To facilitate this process various image processing techniques are studied thoroughly first and then employed to obtain desired results. |
|
MATERIALS AND METHODS
|
|
Data collection and preprocessing
Protocols for kinematic movies with
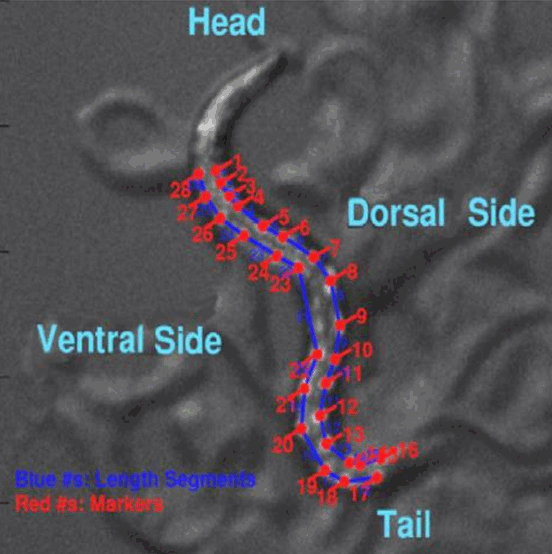
C. elegans 1. C. elegans localization The movies were acquired on clean, smooth agar plates (no food), at room temperature as per the lab protocol. The culture plate was mounted on the stage and focused on worms. Once the worm is located having enough level of GFP (Green fluorescence protein) refection, the capture screen is bypassed to PC screen which gives extra comfort to observe the moving worm on computer and control the stage as it moves out of field. 2. Image Focus and filtering The vital part of video microscopy is localization of animal and focus on individual markers. Exposure settings are fine tuned for capturing all fluorescent markers with optimum brightness and clarity. This will allow us to decide how much distance we can use to have maximum number of oscillations with minimum level of magnification which varies from 50x to 120x. Background light of culture plates were adjusted to rectify edge artifacts. 3. Video Capture Q-imaging capture device captures continuous video at the averageframe capture rate of 1 frame per 0.25 second. This is improved with low exposure and magnification. The video file is saved in the form of data file and time tracking was saved as text file for curve pulse measurements and velocity measurements. On average, one video contains 300–1000 frames. Depending upon the magnitude of speed, oscillations, and preservation of the field, frame number varies in all the videos. The tiff images captured as video frames are finally processed through Matlab algorithm (Figure 1). Our major goal is to understand and evaluate critical behavioral characteristics such as intermarker distance changes, curvature changes and velocity over a defined set of frames captured. |
RESULTS |
|
Wildtype animals were used as the standard for comparison as they show symmetric movements, with no favoring of either axis. The movies were analyzed for L4 wildtype animals with multiple segments of forward and backward locomotion. In analyzing average curvature for wildtype L4 animals, all large peaks displayed in the graphs are during forward movement only. Backward movement data points vary between dorsal and ventral portions with no large peaks on either side. Wildtype (Figure 2) shows the curvature of all markers for each side of the animal, starting from the head. The overall pattern or wave remains the same throughout, and is very similar for points directly next to each other. The stationary segments of the graphs also lie in the trough of the wave, with peaks or crests surrounding on either side. The mean for average dorsal curvature for two separate data sets were –0.015 and –0.006, with a standard deviation of approximately +/- 0.015. It was noted in the curvature graphs that there are more deviations incurvature values for forward movement than for backward. The stationary segment of the graph for average ventral curvature shows all data points on the ventral side at approximately -0.03 (Figure 2). In comparing graphs of average curvature for both data sets, the forward locomotion shows some intense peaks on both dorsal and ventral sides, while backward movement shows trivial and gradual changes in marker positions. In contrast cnd-1 shows more activity in markers near head and tail on dorsal side as well as central markers on ventral side (Figure 3). This coordination can create dorsal asymmetry which is consistent with the observation under microscope. There is little contrast between different dorsal markers, while the individual ventral markers show much more variation with some presenting fairly smooth movement and few markers displaying multiple intense peaks. In cnd-1 L4/adults, datasets were analyzed consisting of both forward and backward movements. Movies for Data sets were 60-120 seconds in length. In observing all the segments of cnd-1 movies, curvature graphs mostly show points on the dorsal side of the body axis, at a distance far from the zero line. Moreover, it would be more interesting to appreciate whether these data can differentiate neural mechanics behind phenotypes. During backward movement, reduction of dorsal inhibition due to absence of DD motoneurons and presence of normal dorsal excitation creates dorsal asymmetry in cnd-1. In contrast, unc-4 shows dorsal asymmetry because of reduction of ventralexcitation, resulting from the removal of interneurons input (gap junction) to VA motoneurons. Computations of experimental graphs for cross sectional distance changes at a particular portion of body are shown here (Figure 4). cnd-1 (Figure 4A) shows low frequency and low peaks of sectional variations in general, however unc-4 (Figure 4B) shows more frequent and high peaks of cross body sectional movements. The genotypic information can be further analyzed to correlate with quantitative phenotypic behavior. |
|
|
|
|
|
|
|
|
DISCUSSION |
|
Based upon the graphical analysis in correlation with the movie timesheets and still images, we can perceive the amount of curvature in cnd-1 mutants both in forward and backwards as dorsal curves. When the animal changes direction in their path, varying heights of peaks are obtained depending on the degree of turn they are making. In observing the wildtype data, the waves of curvature are generally mild and do not form intense peaks or patterns. This quantitative datagraphics enables us for appropriate standard of comparison against any asymmetric mutants. It is revealed quantitatively that wildtype animals are having much smaller units of differences among peak ventral and peak dorsal movements. In comparing the highest peaks for average dorsal curvature of L4 wildtype versus L4 cnd-1 mutants, the large differences in maximum and minimum peak levels is the result of cnd-1 dorsal coiling phenotype. It was impractical to conduct this study in L1 stage as the fluorescence was quite ambiguous and movements are very gradual. It would be interesting if collective data from different labs can be compiled to create comprehensive database of quantitative information of wildtype and mutant forms. As the previous observations mentioned so far indicates that cnd-1 does not act as a binary switch gene [9]. Nor does cnd-1 specify a particular type of motor neurons because DA, DB and DD neurons are equally affected. However, more experimental analysis of phenotypes by qualitative and quantitative methods are needed. As per our lab observations, cnd-1 is ventrally asymmetric in L1 stage and dorsally asymmetric in L4 stage. The change in asymmetric patterns is the result of remodeling of synaptic pattern. It is also observed that cnd-1 is asymmetric during both forward and backward movements and it moves in both directions without any affinity. With fluorescent microscopic studies of L4 cnd-1 mutants with flip13 reporter gene for neurons, it is consistently found that DA motoneurons are disrupted in their position in relation to wildtype. In order to produce dorsal asymmetry in L4, four possibilities can be described: 1) Increased dorsal excitation 2) Decreased ventral excitation 3) Increased ventral inhibition 4) Decreased dorsal inhibition. As cnd-1 affects only embryonic motor neurons, the potential neurons involved could be DA, DB or DD. As DA motoneurons are responsible for backward and DB motoneurons are responsible for forward locomotion behavior, only DD motoneurons could be liable for the asymmetry changes. During L1 stage it is found to have synaptic innervations on ventral side. Vice versa, after molting synaptic innervations shits to dorsal side, it leads to dorsal inhibition. Absence of this inhibition is consistent with the observational studies depicting enhanced dorsal curvature changes during both forward and backward movements. Ventral cord motoneurons are consistently present in cnd-1 and previous studies have shown that cnd-1 gene mutation reduced the number of postembryonic ventral cord motoneurons [10] . Evidently, DD motoneurons are affecting the final phenotypic outcome of cnd mutant forms and our quantitative analysis supports this novel information. Kinematic movies and MATLAB analysis are accurate for quantitative measurement of asymmetric movements of C.elegans cnd-1 mutants. Using mathematical measures for curvature, length and velocity, we can more precisely analyze and verify predictions about other mutant phenotypes and sort mechanisms of their neural circuitry. In addition, this system will facilitate high-throughput collection of quantitative data that can ultimately be used to generate a comprehensive database of C. elegans phenotypes information. |
|
CONCLUSION
|
|
The tools developed in this study for phenotypic analysis will provide reliable, comprehensive statistics of a wide range of locomotion behavioral abnormalities, and will make it possible to standardize assays such that behavioral data of different mutant forms can readily be compared. Such information can potentially benefit future discoveries of genetics behind diseases and potential drug development. |
FUTURE DIRECTIONS |
|
As a technical advancement, to capture maximum path into the imaging system in a single setting, a motorized stage can be used to automatically follow the animal’s movements and hold it in the microscope’s visual field. If a system can be developed to standardize X and Y coordinates in entire movie, we can gather even larger pool of data for a significant duration of locomotion which might be missing in current small scale movies. The detection process can still be improved to allow statistical analysis of population data as well as single cells, to be determined automatically. To study behavioral characteristics, simultaneous reading of multiple data files of different worms collected under different scenarios, e.g. presence and absence of food, chemotaxins, vibration etc. for comparing their various locomotion parameter results in parallel. This can also be applied to compare the locomotion characteristics of same worms in different experiments. Matlab algorithm creates a large pool of data which calls to prepare a database which can be used to store all existing and new data. It can also give an opportunity of ready availability of data for comparison of results of analysis in same lab or in between different labs across the world. |
|
REFERENCES
|
|
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Keyur Vora – Substantial contributions to conception and design, Acquisition of data, Drafting of article, Revising it critically for important intellectual content, Final approval of the version to be published Bill Walthall – Substantial contributions to conception and design, Acquisition of data, Drafting of article, Final approval of the version to be published Gennady Cymbalyuk – Substantial contributions to conception and design, Acquisition of data, Drafting of article, Final approval of the version to be published |
|
Guarantor of Submission
The corresponding author is the guarantor of submission. |
|
Source of Support
None |
|
Consent Statement
Written informed consent was obtained from the patient for publication of this study. |
|
Conflict of Interest
No affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. |
|
Copyright
© 2018 Keyur Vora et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|