|
|
Original Article
| ||||||
| Viral load suppression as a surrogate marker of treatment success in patients on antiretroviral therapy in Namibia | ||||||
| Daniella C Mouton1, Martin Gonzo2, Munyaradzi Mukesi3 | ||||||
|
1Bachelor of Science, Graduate, Health Sciences department, Namibia University of Science and Technology, Windhoek, Namibia.
2Master of Science, Lecturer, Health Sciences department, Namibia University of Science and Technology, Windhoek, Namibia. 3Master of Science, Lecturer, Health Sciences department, Namibia University of Science and Technology, Windhoek, Namibia. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Mouton DC, Gonzo M, Mukesi M. Viral load suppression as a surrogate marker of treatment success in patients on antiretroviral therapy in Namibia. Edorium J Biomed Sci 2017;2:1–8. |
|
Abstract
|
|
Aims:
The study aimed to establish viral load suppression in patients on different antiretroviral regimens in Namibia.
Methods: This was a retrospective cross-sectional study conducted at the Windhoek Central hospital (WCH) antiretroviral therapy (ART) clinic from 2014–2015. The study population included 302 patients on different ART regimens during the period of 2014–2015. Results: Out of these patients 225 were females (74.5%) and 77 were males (25.5%). The age range of the respondents was 16–73 years old with a mean age of 34.6 years old. Most of the patients (93%) had good adherence to treatment. The majority of the patients (93.9%) on ART achieved viral load suppression after six months, with 93.3% of the patients maintaining suppression of the viral load after 12 months. Most of the patients on TDF/3TC/EFV (95.2%) suppressed viral load after 12 months. The regimen associated with the highest number of patients (n=5) who failed viral load was TDF/3TC/EFV. Conclusion: Viral load suppression in Namibian patients was better than results recorded in the United States of America and other countries in Sub-Saharan Africa. | |
|
Keywords:
Antiretroviral therapy, Namibia, Suppression, Viral load
| |
|
Introduction
| ||||||
|
The human immunodeficiency virus (HIV) is a virus which attacks the CD4 T-helper cells leading to immunosuppression [1] . When the virus has managed to suppress the host's immune system to below 200 CD4 cells/µL, the patient is said to have acquired immunodeficiency syndrome (AIDS). In order to move towards the aim of ending the AIDS epidemic by 2030, World Health Organization (WHO) has implemented new guidelines whereby all patients who are HIV positive are put on antiretroviral therapy (ART) [2][3]. The 90-90-90 target which is to be achieved by 2020 is a plan which seeks to have 90% of the population knowing their status, 90% of those that tested positive having access to treatment and 90% of those on treatment having achieved viral load suppression [4]. The aim of ART is to achieve viral suppression. Viral load suppression is defined as two viral load measurements below 1000 copies/ml within 3–6 months [5]. According to United Nations programme on HIV/AIDS (UNAIDS), an average of 36.9 million people were infected with HIV in 2015, with 17 million of those on ART [4]. In 2015, it was recorded that 2.1 million patients were newly infected with 1.1 million AIDS-related deaths worldwide [4]. The region with the highest HIV burden is East and Southern Africa, with 52% of all patients who live with HIV. West and central Africa encompass 17.7% of the patients who live with HIV, followed by the Asia and Pacific with 13.9%. Six point five percent of people living with HIV live in western and central Europe and North America. Latin America and the Caribbean's make up 5.4% of patients living with HIV [4]. Eastern Europe, northern Africa, the Middle East make up the remaining 4.5% [4]. More HIV positive people live in middle to low income countries, with the larger majority living in Africa [6] . Around 69% of all people living with HIV around the world live in Africa [6]. In Africa, an estimated 25 million people are infected with HIV, with 12 million of those receiving ART [7]. According to the WHO, 19 million of the infected people live in Eastern and Southern Africa, with 10 million of them receiving ART [4]. In Namibia, WHO reported that and estimated 210, 000 people were infected with HIV, with 3100 AIDS related deaths in 2015 [4]. In Namibia, the prevalence of HIV infection has steadily decreased from 22% in 2002 to 16.9% in 2014 [8]. In 2013–2014, 81% of patients over the age of 15 years old were on ART, and 77.5% of those patients were still on ART after 12 months. Antiretroviral therapy and drug resistance Tenofovir is a nucleoside reverse transcriptase inhibitor (NRTI) which is recommended for first-line ART [5]. In studies done in, Europe, Latin and North America, Asia and Sub-Saharan Africa, the area with the highest prevalence of tenofovir (TDF) resistance (57%) was Sub-Saharan Africa. Europe followed Sub-Saharan Africa with 20% TDF resistance in patients who had failure to suppress viral [12]. The frequency of resistance was as high as 11.6% in ARV-naive patients in Uganda [11]. Uganda introduced ARVs early compared to other African countries such as Kenya, Nigeria, South Africa, Zambia and Zimbabwe. PharmAccess Africa discovered an annual increase in the prevalence of ARV-resistance since the initiation of ARV; in Southern Africa by 14%, 29% in East Africa and 3% in West and Central Africa [11]. A study done in Gabon showed that 41.3% of the patients had virological failure. Out of the patients who had virological failure, 57.7% had more than 1 drug-resistant mutation [13]. In order to reach viral load suppression patients should be adherent to their treatment ≥95% of the time [9]. In a study done to compare failure to suppress viral load with adherence, patients who were ≥95% adherent to treatment only 22% had treatment failure. In patients who were only adherent to treatment 90–94.9% of the time, treatment failure was reported at 55% and in patients who adhered to treatment 80–89% of the time, 67% had treatment failure [9]. This shows a direct relationship between poor adherence and failure to suppress viral load. Adherence to medication is affected by; adverse effects of the ARTs, complex dosing regimens and dietary restrictions especially in third world countries. In addition, the following factors concerning health care facilities also play a role; long distances to the ART clinics, long waiting times at the clinics, costs and stigma from health workers and the community [14]. It should, however, be noted that poor adherence is not the only determinant for treatment failure. Other factors include malabsorption, drug metabolism, very low baseline CD4 counts, prior drug resistance, and concurrent opportunistic infections [8] [9]. Antiretroviral monitoring Antiretroviral therapy is monitored using three main methods clinical, immunological and virological monitoring. Clinical monitoring involves classifying the stage of disease according to the symptoms the patient presents with, the infections increase with severity as the disease progresses. Clinical stage one is the primary stage of infection, patients may be symptomatic or present with flu-like symptoms a few weeks after infection [17]. Clinical stage 2 presents with moderate unexplained weight loss of less than 10% of total body weight, recurrent upper respiratory infections, oral ulcers and fungal nail infections. Clinical stage 3 involves unexplained weight loss of more than 10% of total body weight, unexplained fever and unexplained chronic diarrhea lasting more than a month. Clinical stage 4 is the last clinical stage. Symptoms include, HIV wasting syndrome, pneumocystis pneumonia, recurrent severe bacterial pneumonia, Kaposi's sarcoma, invasive cervical cancer, chronic isosporiasis and central nervous system toxoplasmosis [17]. Clinical failure is characterized through a new or recurrent clinical event which indicates a failing immune system after six months of treatment success [5]. Immunological monitoring involves monitoring treatment through CD4 count measurements. If the CD4 count is maintained or increases the treatment is presumed successful. Immunological failure is characterized by a CD4 count below 250 cells/µL or persistent measures of 100 cells/µL [5]. Some countries in the Asia Pacific region rely on clinical and immunological monitoring to determine treatment failure [18]. This is not an accurate measure and leads to the development of drug resistant mutations as virological failure is not ascertained soon enough. Virological failure is characterized as two consecutive measures of HIV viral load above 1000 copies/ml within 3–6 months, supposing adherence counseling followed the first measurement [5]. The treatment failure of South Africa where viral load is monitored routinely was compared to Malawi and Zambia where immunological monitoring is done routinely. The frequencies of prolonged treatment failure in Malawi and Zambia were 3.7% and 3.6–3.9% respectively. This was higher in comparison to South Africa where the frequency was 1.3% [17]. Viral load monitoring is important as CD4 cell count is not sufficient in measuring treatment success. If treatment success cannot be ascertained early, the patient remains on the treatment which leads to extensive NRTI cross-resistance [19]. Measurement of treatment success through viral load measurement also prevents unnecessary switches to second-line treatment. Second-line treatment is more expensive, and once resistance is formed against second-line treatment, treatment options are limited for the patient [19]. Drug resistance in Africa is largely due to the lack of viral load monitoring, treatment interruptions due to stock delays, drug interactions and inferior antiretroviral regimens [11]. With monitoring of ARV success, treatment failure can be picked up soon and the patient placed on an alternative ARV regimen. Through regular viral load monitoring the adherence to treatment can also be ensured [11]. The new ARV guidelines stating that a patient should go on treatment as soon as possible after diagnosis increase the chances of drug resistant mutations as seen in a study done in Africa [11]. The large rate of replication of HIV along with the lack of exonuclease proof-reading activity makes HIV highly susceptible to mutations. These mutations cause resistance to the ARV drugs. In the USA and Europe, patients are tested for genotypic drug resistance in order to select the correct treatment regimen. This, however, is not possible in third world countries such as Namibia due to the high cost surrounding such tests [20]. Combination treatments are given to patients in order to prevent resistance and reduce viral replication [21]. Formation of drug resistance is dependent on factors such as the relative fitness of the HIV variants and interactions of the mutations creating cross-resistance, the genetic barrier to resistance and ART half-lives [15]. In patients who had treatment failure 56.7% had at least one drug resistance mutation in semirural Gabon [13].Thymidine analogue mutations (TAMs) are formed as a result of continued use of the ART after prolonged virological failure. AZT and NVP are at higher risk of TAMs than EFV which would explain why WHO recommends EFV over NVP as a first line NNRTI [3] [15]. The duration of virological failure increases the number of mutations formed. However, mutations against TDF/(3TC or FTC)/(EFV or NVP) are predictable. The mutations would lead to NVP or EFV resistance, 3TC/FTC resistance and in prolonged virological failure it would lead to TDF resistance [15]. A study in Gabon showed that 37.6% of patients who had virological failure had dual-class resistance to NRTIs and NNRTIs [13]. A global study showed that 3TC and FTC had decreased genetic resistance barrier due to the fact that changes in only one amino acid is required for drug resistance to follow [12]. LPV/r is a good PI for a second-line treatment, because it is effective regardless of existing NRTI mutations. This could be due to a high genetic barrier to resistance of the drug [15]. Resistance to first-line NNRTIs can be acquired through previous use of prevention of mother-to-child transmission (PMTCT) [11] . The chances of treatment failure and the acquisition of drug resistance is increased against NNRTIs when the mother has used PMTCT [11]. Resistance has been reported in 60% of infants under six months whose mothers were on PMTCT that failed [11]. Children who take two or three drugs ART regimen report less than 12% drug resistance. Ritonavir (protease inhibitor) boosted regimens show more promising results than the previously used lopinavir-boosted regimens, but lack practicality as the ritonavir-boosted regimens have poor palatability, inconvenient formulation and require cold-chain [8]. | ||||||
|
Materials and Methods
| ||||||
|
Research study design Inclusion and exclusion criteria Data collection procedure and analysis Ethical considerations | ||||||
|
Results | ||||||
|
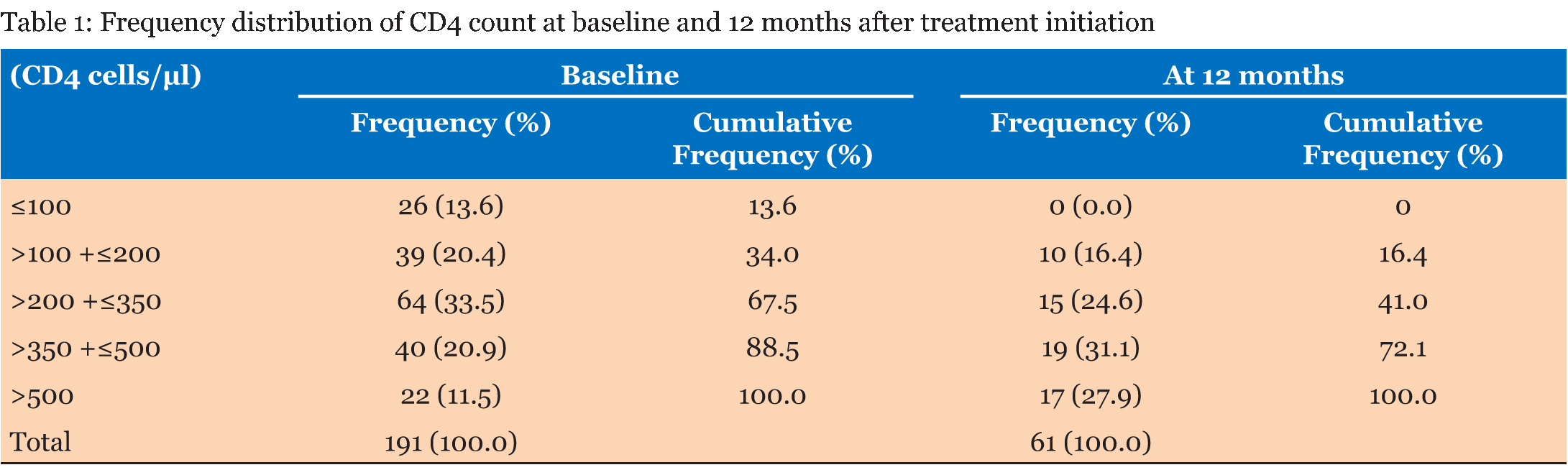
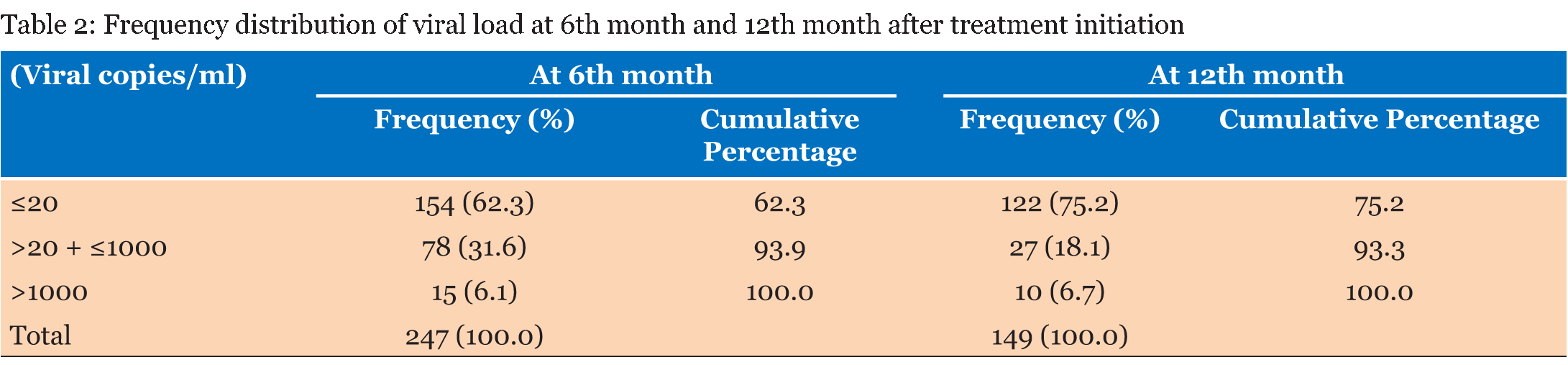
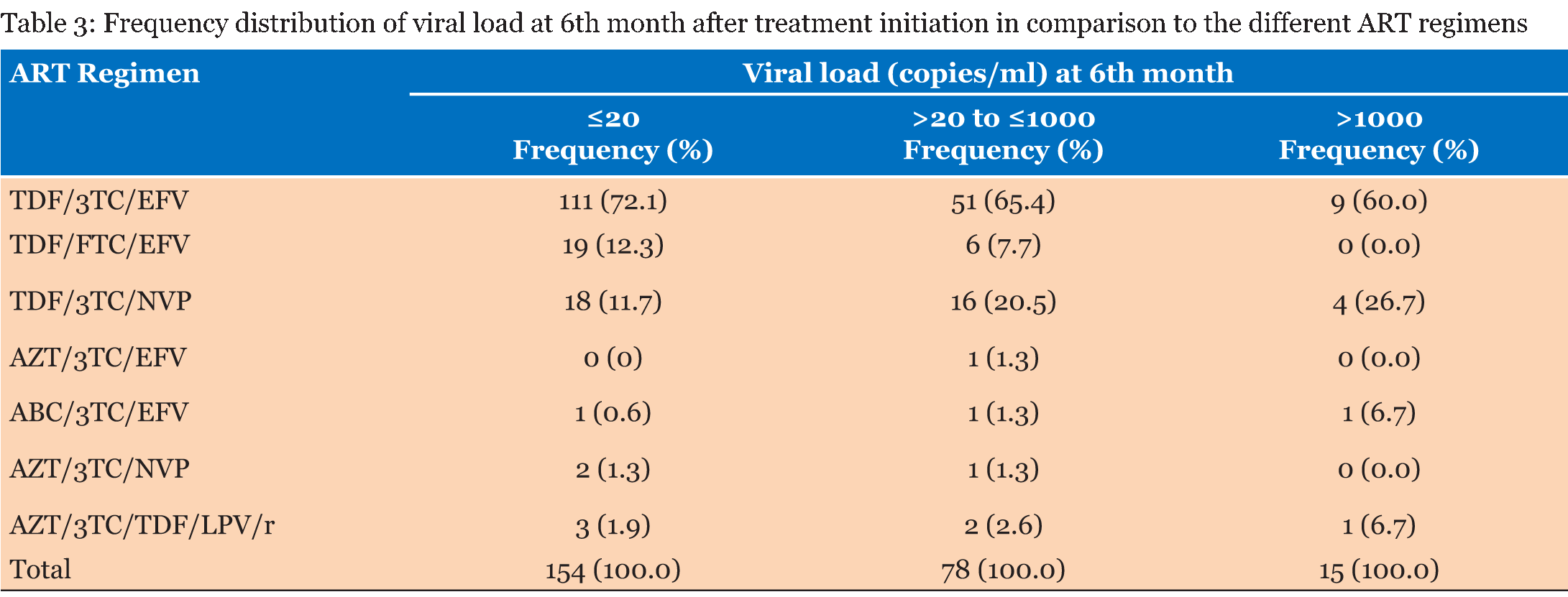
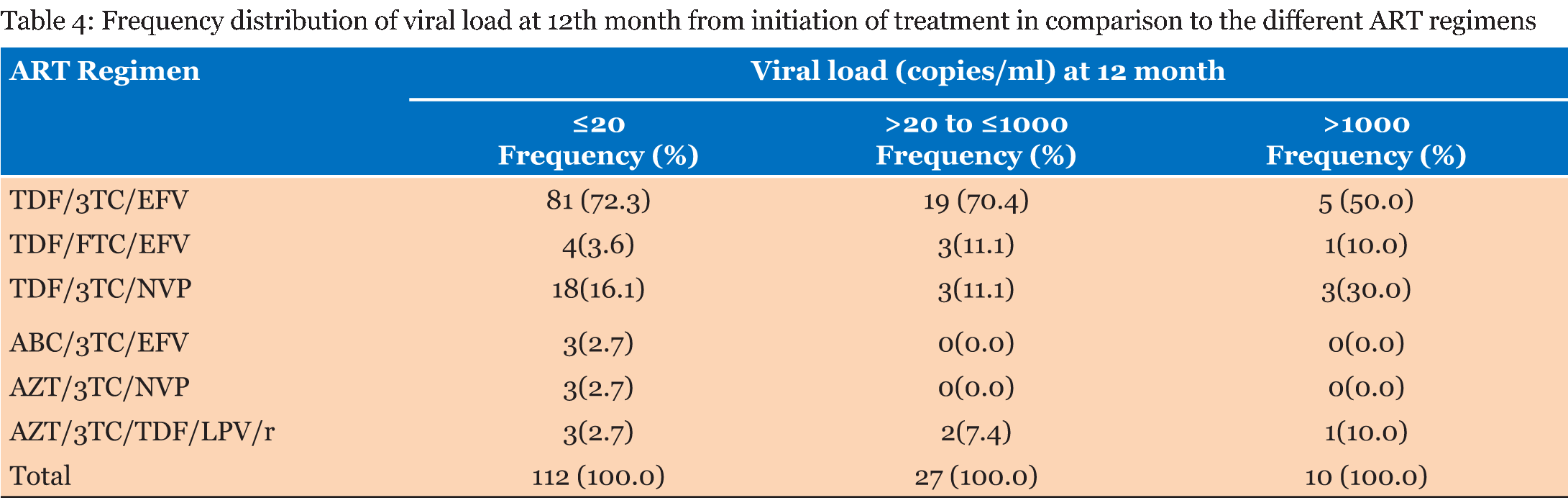
The study population consisted of 302 patients who were registered to receive their ART at the Windhoek Central Hospital ART clinic. Out of these patients 225 were females (74.5%) and 77 were males (25.5%). A total of 132 (58.7%) of the females started treatment while they were pregnant or became pregnant during the study period. The age range of the subjects was 16–73 years old with a mean age of 34.6 years old. The range of the baseline CD4 cell count was 5–1546 cells/µL with a mean of 303 cells/µL. The range of the CD4 count after 12 months was 112–987 cells/µL with a mean of 421 cells/µL. The range of the baseline viral load was from 28.6–688 869.5 copies/ml and the mean viral load was 41 553.3 copies/ml. Most of the patients (93%) had good adherence to treatment. Table 1 gives the frequency distribution of CD4 count at baseline and 12 months after treatment initiation. Most of the patients started ART with a CD4 cell count of >200 to ≤350 cells/µL. Eighty-nine percent of the patients had a CD4 cell count ≤500 cells/µL. After 12 months all the patients had CD4 cell counts >100 cells/µL. The majority of the patients had a CD4 cell count of >350 to ≤500 cells/µL 12 months after treatment initiation. Table 2 presents the frequency distribution of viral load at six months and 12 months after treatment initiation. The majority of the patients (93.9%) on ART achieved viral load suppression after six months, with 93.3% of the patients maintaining suppression of the viral load after 12 months. The frequency distribution of viral load at 6 months after treatment initiation in comparison to the different ART regimens is given in Table 3 . All the patients that were on TDF/FTC/EFV had their viral loads suppressed after six months, in addition 76% of these patients had viral load suppression below <20 copies/ml. Ninety-four percent of the patients on TDF/3TC/EFV had viral load suppression after six months. TDF/3TC/EFV had the highest number of patients (n=9) who failed to suppress viral load. Table 4 gives the frequency distribution of viral load after 12 months from initiation of treatment in comparison to the different ART regimens. Most of the patient on TDF/3TC/EFV (95.2%) suppressed viral load after 12 months. The regimen associated with the highest number of patients (n=5) who had failure to suppress viral load was TDF/3TC/EFV. | ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
In order to achieve viral load suppression, good adherence, defined as adhering to treatment =95% of the time, is important [8]. The benefits of good adherence further spread to the reduction in transmission of HIV in patients who have achieved viral load suppression and the formation of drug resistant mutations is significantly decreased [22]. In this study, it was found that the majority (93.4%) of the patients on ART had good adherence to treatment. This is very good in comparison to a study done in Lao Peoples's Democratic Republic in which only 59.5% of the patients on ART were adherent to treatment [22]. In Table 1, the majority of patients (33.5%) had a baseline CD4 cell count between 200 and 350 cells/µL. A study done in China showed a median baseline CD4 count of 294 cells/µl. This is within the range of 200–350 cells/µl as seen in this current study [23]. A study done in Ethiopia showed that 64.4% of the patients in the study had baseline CD4 cell counts less than 200 cells/µL [24] . However, it should be noted that in this study ART was initiated with a CD4 cell count below 350 cells/µL as opposed to 500 cells/µL. After 12 months from initiation of ART none of the patients had CD4 cell counts below 100 cells/µL and most of the patients (31.3%) had CD4 cell counts between 350 and 500 cells/µL. In a study done in Ethiopia, the median CD4 cell count after 12 months was 300 cells/µL [24]. The difference could be attributed to the fact that in Ethiopia the CD4 cell count for starting ART was much lower than the current study. A study done in Nigeria showed a CD4 count of 170 cells/µl after 12 months, the low CD4 count compared to this study could be due to the fact that adherence was lower (78.6%) than this study [25]. The range of the baseline viral load was from 28.6 to 688, 869.5 copies/ml and the mean viral load was 41 553.3 copies/ml, however this was only out of 21 patients. In Table 2, the frequency of patients that had viral load suppression after six months, according to the WHO guidelines (2015), was 93.9% and after 12 months it was 93.3%. This is higher in comparison to a study done in the United States where only 77% viral load suppression according to the WHO guidelines was reported [26]. A study in Nigeria showed viral load suppression of 90%, which is lower than this study [27]. Another study done in Botswana showed viral load suppression of 70.2% [28]. The aim of the MOHSS, Namibia was to suppress viral load to <20 copies/ml [8]. The frequency of viral load suppression to below 20 copies/ml was 62.3% after six months and 75.2% after 12 months. In 18 countries in Sub-Saharan Africa, a study showed that 78% of patients had viral load suppression after six months, and 76% had viral load suppression after 12 months [29]. This is lower than noted in this current study. This current study could have higher viral load suppression due to high adherence. The treatment with the highest frequency of treatment failure (n = 9) was TDF/3TC/EFV. The treatment regimen with the second highest frequency (n = 4) of treatment failure was TDF/3TC/NVP. TDF/FTC/EFV, AZT/3TC/EFV and AZT/3TC/NVP showed no treatment failure. It should, however, be noted that the majority of the patients (69.2%) on treatment in this study were on TDF/3TC/EFV. This was followed by patients on TDF/3TC/NVP, who made up 15.4% of the sampled population at six months after initiation of treatment as given in Table 3. Patients on TDF/FTC/EFV made up 10.1% of the sampled population and no virological failure was observed in this treatment regimen. The ART regimen most commonly associated with failure to suppress viral load after 12 months was TDF/3TC/EFV (50%). Thirty percent of patients with virological failure after 12 months were taking TDF/3TC/NVP. TDF/FTC/EFV and AZT/3TC/TDF/LPV/r each made up 10% of the patients who experiences virological failure. In a study done in Cambodia, most of the patients (89%) on LPV/r based second line regimens had virological suppression after 12 months [18]. ABC/3TC/EFV and AZT/3TC/NVP had no virological failure. | ||||||
|
Conclusion
| ||||||
|
The antiretroviral therapy regimen most commonly associated with failure to suppress viral load is TDF/3TC/EFV, followed by TDF/3TC/NVP. After 12 months TDF/3TC/EFV was still most commonly associated with failure to suppress viral load. This is followed by TDF/3TC/NVP. Most of the patients suppressed viral load six months after initiation of treatment. This was higher compared to other African countries and the USA. | ||||||
|
Abbreviations
| ||||||
|
AIDS Acquired Immunodeficiency syndrome | ||||||
|
Acknowledgements
| ||||||
|
We would like to acknowledge Windhoek Central Hospital for their assistance in the research. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Daniella C. Mouton – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Final approval of the version to be published Martin Gonzo – Substantial contributions to conception and design, Revising it critically for important intellectual content, Final approval of the version to be published Munyaradzi Mukesi – Substantial contributions to conception and design, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2017 Daniella C. Mouton et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|